REGULATION (EU) 2025/1731: UPDATE OF REACH ANNEX XVII WITH NEW CMR 1B SUBSTANCES
The European Union is strengthening protection against hazardous chemicals. Following the amendments specified by Commission Delegated Regulation (EU) 2024/197, which updates Annex VI of the CLP Regulation as part of the 21st ATP Technical Adaptation, the EU is introducing into Appendices 2, 4 and 6 of Annex XVII to Regulation (EC) No 1907/2006 (REACH) the substances newly classified as category 1B CMR. Here’s what you need to know.
On 8 August 2025, the European Commission adopted Regulation (EU) 2025/1731, amending Regulation (EC) No 1907/2006 (REACH) regarding substances classified as carcinogenic, germ cell mutagenic, or toxic for reproduction (CMR) category 1B. This REACH regulatory update aims to strengthen the protection of human health and the environment by introducing stricter restrictions on the placing on the market and use of such substances in products accessible to the general public.
What does this amendment imply?
Regulation (EU) 2025/1731 introduces changes to Appendices 2, 4 and 6 of Annex XVII REACH, adding new substances classified as CMR category 1B. These substances will be subject to specific restrictions, such as the ban on their marketing and use in consumer products. The restrictions will apply as of 1 September 2025, although operators may choose to implement them earlier.
In addition, the Regulation makes an amendment to Appendix 11, adding exceptions for certain substances.
Amendments
Annex XVII to Regulation (EC) No 1907/2006 is amended as follows:
In Appendix 2, the following entries are inserted in the table, in accordance with the order of the classification numbers set out therein:
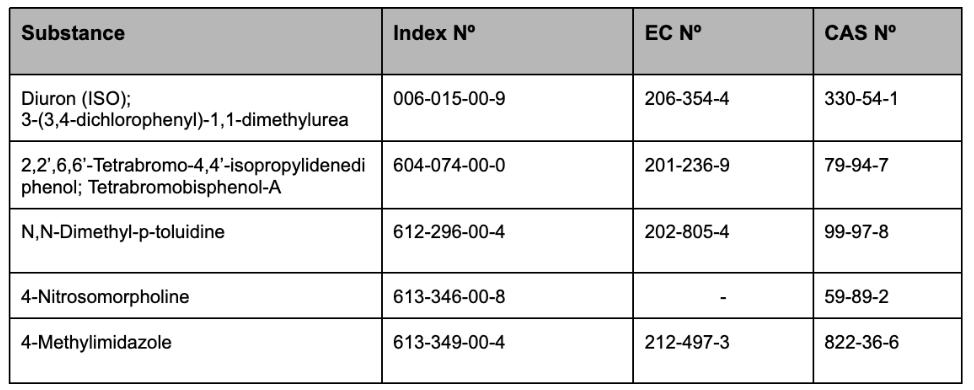
In Appendix 4, the following entry is inserted in the table, in accordance with the order of the classification numbers set out therein:
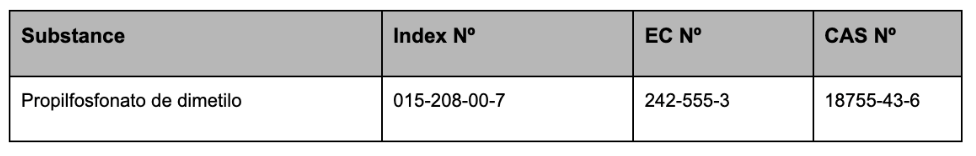
In Appendix 6, the following entries are inserted in the table, in accordance with the order of the classification numbers set out therein:

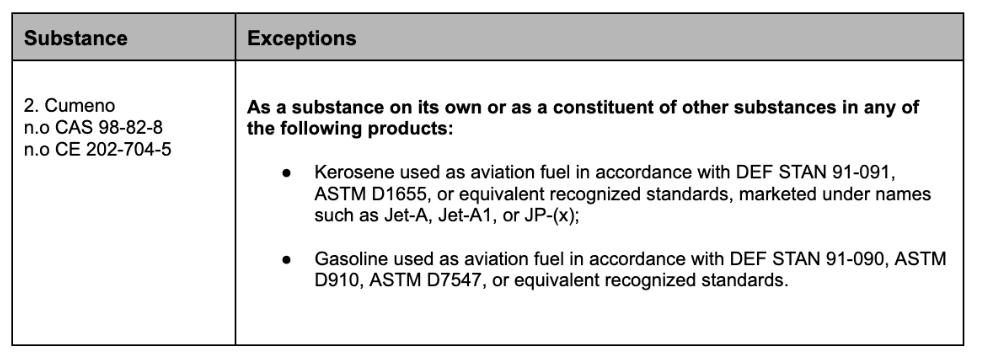
In Appendix 11, the following text is added:
Expected Impact
This measure reinforces the European Union’s commitment to protecting public health and the environment, aligning with scientific advances in the classification of hazardous substances. The implementation of these restrictions will help reduce consumer exposure to harmful chemicals, promoting a safer and healthier environment.
For more information, you can consult the full text of Regulation (EU) 2025/1731.
Next Steps
Regulation (EU) 2025/1731 strengthens Europe’s commitment to chemical safety and sustainability. For companies, this means the need to adapt processes, safety data sheets, and compliance assessments before September 2025.
You can access the full text of Regulation (EU) 2025/1731 on EUR-Lex to verify the restrictions in detail.
At eQgest, we closely monitor these regulatory updates to ensure our clients have software that is always up to date in matters of REACH, CLP, safety data sheets, and chemical regulation management.
Contact us here to learn how our software can help you stay always up to date.
Receive regulatory updates straight to your inbox
Newsletter with updated chemical regulations, regulatory news from the sector, and upcoming webinars — carefully selected for professionals like you.




